The examination of the Fischer esterification mechanism continues to reveal its intricacies and practical applications. This reaction, a cornerstone of organic chemistry, enables the synthesis of esters, compounds with diverse uses in industries ranging from food to pharmaceuticals.
Understanding the mechanism and factors influencing the Fischer esterification reaction is crucial for optimizing its efficiency and expanding its applications. Recent advancements have shed light on alternative catalysts and reaction conditions, while computational chemistry has provided insights into the reaction pathway.
Mechanism of the Fischer Esterification Reaction
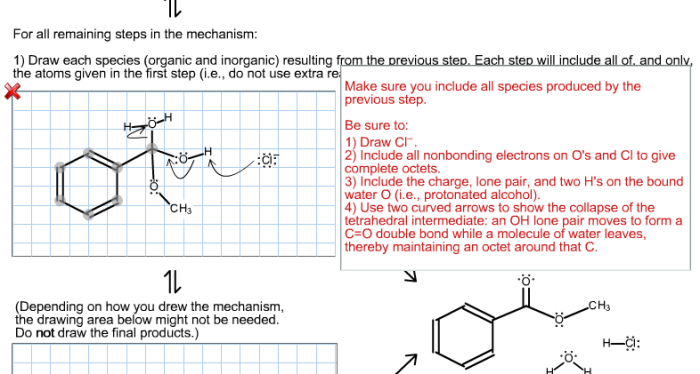
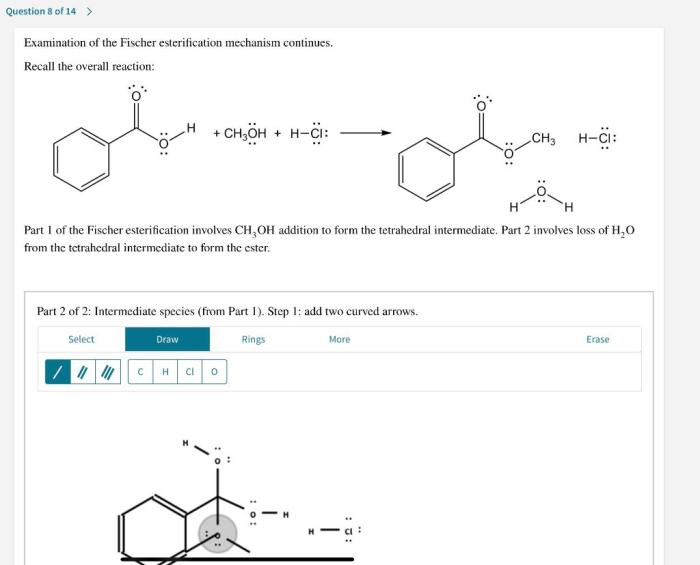
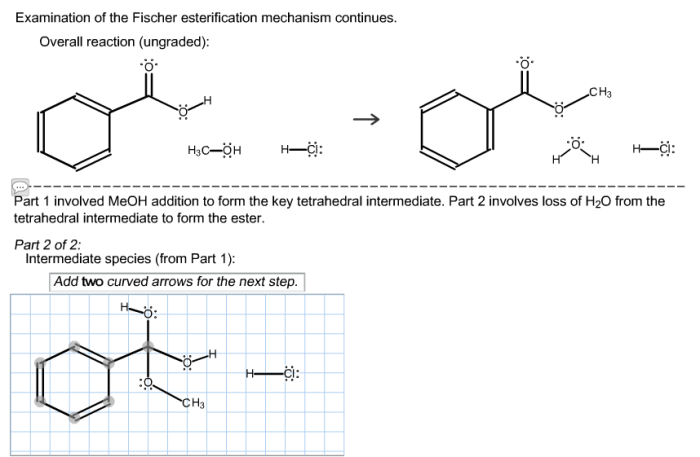
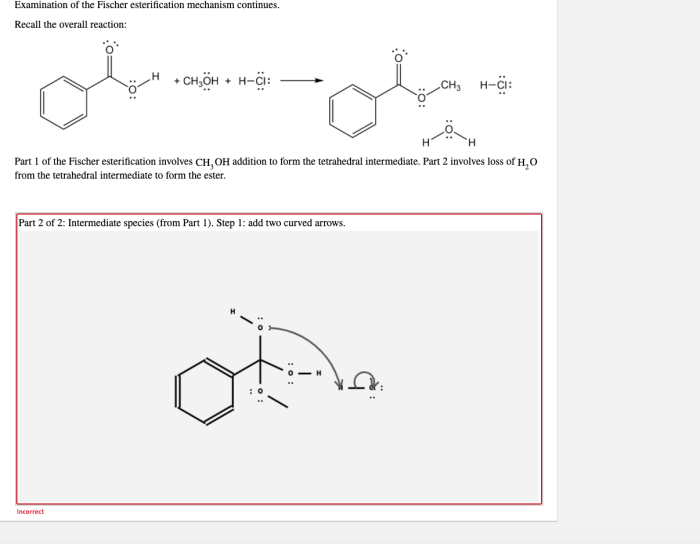
The Fischer esterification reaction is a chemical reaction that involves the conversion of an alcohol and a carboxylic acid into an ester. The reaction is typically catalyzed by an acid, such as sulfuric acid or hydrochloric acid. The mechanism of the reaction is as follows:
- The acid catalyst protonates the carboxylic acid, making it more electrophilic.
- The alcohol attacks the electrophilic carboxylic acid, forming a tetrahedral intermediate.
- The tetrahedral intermediate collapses, expelling a water molecule and forming the ester.
The rate of the Fischer esterification reaction is affected by several factors, including the concentration of the reactants, the temperature of the reaction, and the type of acid catalyst used.
Applications of the Fischer Esterification Reaction
The Fischer esterification reaction is a versatile reaction that is used in the synthesis of a wide variety of esters. Esters are important compounds that are used in a variety of industries, including the food, pharmaceutical, and fragrance industries.
The Fischer esterification reaction has several advantages over other methods of ester synthesis. These advantages include:
- The reaction is relatively simple to perform.
- The reaction is efficient and produces high yields of the desired product.
- The reaction is tolerant of a wide range of functional groups.
However, the Fischer esterification reaction also has some disadvantages. These disadvantages include:
- The reaction is slow and can take several hours or even days to complete.
- The reaction is not very selective and can produce a mixture of products.
- The reaction can be corrosive and can damage equipment.
Modifications of the Fischer Esterification Reaction
Several modifications have been made to the Fischer esterification reaction to improve its efficiency and selectivity. These modifications include:
- Using a more active acid catalyst, such as p-toluenesulfonic acid or trifluoroacetic acid.
- Using a Dean-Stark trap to remove the water produced during the reaction.
- Using a microwave or ultrasound reactor to accelerate the reaction.
These modifications can significantly improve the yield and selectivity of the Fischer esterification reaction.
Recent Advances in the Study of the Fischer Esterification Reaction
Recent research has led to a better understanding of the Fischer esterification reaction. This research has focused on the use of computational chemistry to model the reaction pathway and on the development of new methods for monitoring the progress of the reaction.
Computational chemistry has been used to study the transition state of the Fischer esterification reaction. This research has shown that the transition state is a concerted process in which the proton transfer and the nucleophilic attack occur simultaneously.
New methods for monitoring the progress of the Fischer esterification reaction have also been developed. These methods include the use of NMR spectroscopy and IR spectroscopy.
Applications of the Fischer Esterification Reaction in Green Chemistry
The Fischer esterification reaction can be modified to make it more environmentally friendly. These modifications include:
- Using a biocatalyst, such as an enzyme.
- Using a renewable feedstock, such as biomass.
- Using a solventless reaction.
These modifications can reduce the environmental impact of the Fischer esterification reaction and make it a more sustainable process.
Helpful Answers: Examination Of The Fischer Esterification Mechanism Continues
What are the key steps in the Fischer esterification mechanism?
The mechanism involves protonation of the carboxylic acid, nucleophilic attack by the alcohol, formation of a tetrahedral intermediate, and elimination of water to yield the ester.
How does the acid catalyst influence the reaction rate?
The acid catalyst protonates the carboxylic acid, making it more electrophilic and susceptible to nucleophilic attack by the alcohol.
What factors affect the efficiency of the Fischer esterification reaction?
Factors such as temperature, reaction time, solvent, and catalyst concentration can influence the reaction rate and yield.