In the realm of materials science, the concept of “what is considered a good conductor milady” takes center stage, beckoning us to explore the fascinating properties and applications of these materials that facilitate the efficient flow of electricity and heat.
This discourse delves into the defining characteristics, diverse uses, and ongoing advancements in conductor technology, offering a comprehensive understanding of this fundamental aspect of materials engineering.
As we embark on this journey, we shall unravel the factors that govern the conductivity of materials, examining their electrical and thermal properties. We will encounter examples of materials that excel as conductors and delve into their practical applications in electrical systems, thermal management, and various industries.
Introduction

In the realm of materials science, the concept of electrical conductivity plays a pivotal role in determining the efficiency and functionality of electrical systems. Good conductors are materials that possess the ability to facilitate the flow of electric current with minimal resistance, making them essential components in a wide range of applications.
The conductivity of a material is influenced by several key factors, including its atomic structure, electron mobility, and temperature. Understanding these factors is crucial for selecting the most appropriate conductor for specific applications.
Properties of a Good Conductor: What Is Considered A Good Conductor Milady
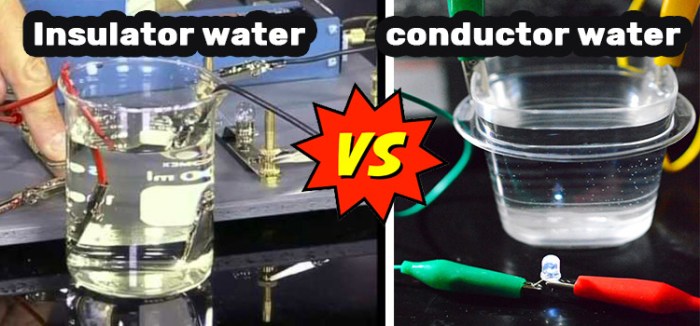
Electrical Properties
Good conductors exhibit exceptional electrical properties, allowing them to readily conduct electric current. These properties include:
- Low electrical resistivity: Resistivity measures the resistance of a material to the flow of electric current. Good conductors have low resistivity, enabling them to conduct electricity with minimal energy loss.
- High electron mobility: Electron mobility refers to the ease with which electrons can move within a material. Good conductors have high electron mobility, facilitating the rapid flow of electric current.
Thermal Properties
In addition to their electrical properties, good conductors also possess favorable thermal properties:
- High thermal conductivity: Thermal conductivity measures a material’s ability to transfer heat. Good conductors have high thermal conductivity, allowing them to efficiently dissipate heat generated by the flow of electric current.
Examples of Good Conductors
Some common examples of good conductors include:
- Copper
- Silver
- Gold
- Aluminum
- Steel
Applications of Good Conductors
Electrical Systems
Good conductors are indispensable in electrical systems, serving as:
- Wires and cables: Conductors are used to transmit electricity from power sources to various electrical devices.
- Busbars: Conductors that distribute electricity within electrical panels and switchboards.
- Capacitors and inductors: Conductors are used as plates or windings in capacitors and inductors, respectively, to store electrical energy.
Thermal Management Systems, What is considered a good conductor milady
Due to their high thermal conductivity, good conductors are also utilized in thermal management systems:
- Heat sinks: Conductors are used as heat sinks to dissipate heat from electronic components.
- Heat exchangers: Conductors are used in heat exchangers to transfer heat between fluids.
Real-World Examples
In various industries, good conductors play a crucial role:
- Electrical power transmission: Copper and aluminum conductors are used in power lines to transmit electricity over long distances.
- Electronics: Gold and silver conductors are used in printed circuit boards (PCBs) to connect electronic components.
- Automotive industry: Copper conductors are used in wiring harnesses and electrical systems of vehicles.
Comparison of Conductors
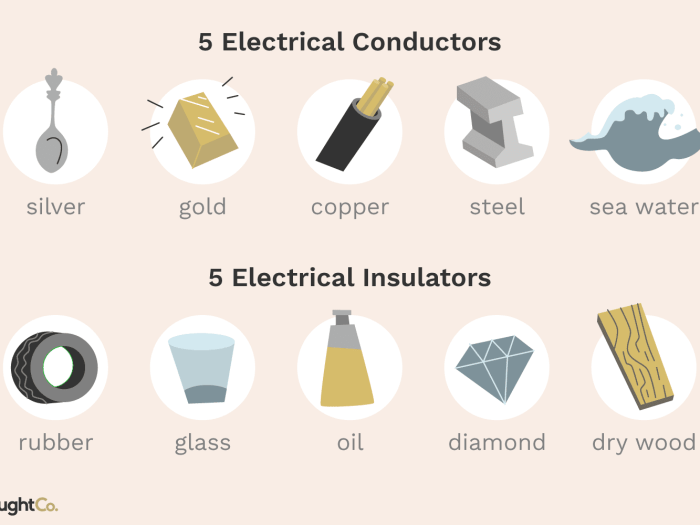
| Material | Electrical Conductivity (S/m) |
|---|---|
| Copper | 5.96 x 107 |
| Silver | 6.30 x 107 |
| Gold | 4.11 x 107 |
| Aluminum | 3.77 x 107 |
| Steel | 1.04 x 107 |
Copper and silver are the most conductive materials, followed by gold and aluminum. Steel has a lower conductivity than the other materials listed.
Advantages and Disadvantages of Different Conductors
Each conductor material has its own advantages and disadvantages:
- Copper: High conductivity, relatively low cost, but can oxidize.
- Silver: Highest conductivity, but expensive and prone to tarnishing.
- Gold: Excellent conductivity, corrosion-resistant, but very expensive.
- Aluminum: Lightweight, low cost, but lower conductivity than copper.
- Steel: Strong, inexpensive, but low conductivity.
The choice of conductor material for a specific application depends on factors such as cost, conductivity, weight, and corrosion resistance.
Future Developments in Conductor Technology
Research is ongoing to develop new materials with even higher conductivity than existing conductors.
Emerging Technologies
- Nanomaterials: Nanomaterials, such as carbon nanotubes and graphene, have shown promise for improving the conductivity of materials.
- Superconductors: Superconductors are materials that exhibit zero electrical resistance at very low temperatures. They have the potential to revolutionize electrical power transmission and other applications.
Potential Applications
New conductor technologies could lead to:
- More efficient electrical power transmission
- Smaller and more powerful electronic devices
- Improved thermal management systems
Challenges and Opportunities
While the development of new conductor technologies holds great promise, there are also challenges that need to be addressed:
- Cost: New conductor materials may be expensive to produce.
- Scalability: It may be difficult to scale up the production of new conductor materials to meet demand.
- Environmental impact: The production of new conductor materials may have environmental consequences.
Despite these challenges, the potential benefits of new conductor technologies are significant, and research is ongoing to overcome these challenges.
Frequently Asked Questions
What are the key factors that determine the conductivity of a material?
The conductivity of a material is primarily influenced by its atomic structure, the number of free electrons available for conduction, and the presence of impurities or defects that can impede electron flow.
Can you provide some examples of materials that are considered good conductors?
Copper, silver, gold, and aluminum are widely recognized as excellent electrical conductors due to their high concentration of free electrons and low electrical resistance.
What are the practical applications of good conductors in electrical systems?
Good conductors are indispensable in electrical systems, serving as wires, cables, and components that facilitate the efficient transmission of electricity for power distribution, electronic devices, and industrial machinery.